



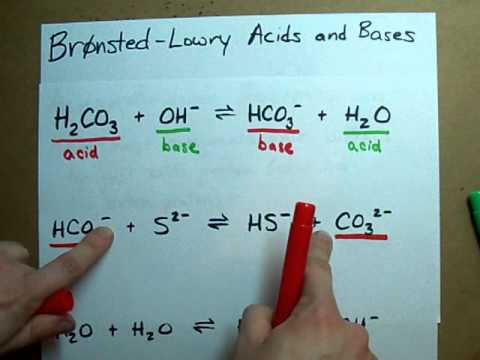




H2SO4 is the first conjugate acid because it donates a proton, which is the H+, to become HSO4-, and the H2O is the first conjugate base because it accepts a proton to become H3O+. H3O+ is the... acid HSO− 4(aq) → H+ (aq) + conjugate base SO2− 4(aq) Once again, notice that the charge is balanced because removing a 1 + charge from a compound that has a 1 − charge will give you a compound that has a 2 − net charge. Answer link. Which is a conjugate acid-base pair in the following equation? H2SO4 + H2O ----> HSO4 -1 + H3O +1 a H2SO4 and H2O b H3O +1 and H2SO4 c HSO4 -1 and H3O +1 d H2SO4 and HSO4 -1 Question:.What Is The Conjugate Base Of HSO4 In The Reaction Below? A) HSO4 B) CO,2 C) OH. RFind The PH Of A 0.183 M Aqueous Solution Of Hypobromous Acid (HOBr), For Which K-2.06 X 10 F) 9.28 Conjugate base. In a reaction of an acid in an aqueous solution, it loses a proton or Hydrogen ion. As the acid releases a proton or Hydrogen ion, it produces a compound that is one proton away or Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. What is the conjugate base of hso4 in the reaction below? Explanation: Although it has a negative charge, it will never accept a H+ to form H2SO4 (sulfuric acid) . That is because sulfuric acid is a strong acid and completely disassociates in water. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. NH4+ is the conjugate acid to the base NH3, because NH3 gained a hydrogen ion to form NH4+.The conjugate base of an acid is formed when the acid donates a proton. Problem: Identify the conjugate acid of CO32– and the conjugate base of HSO4– in the following reaction:HSO4–(aq) + CO32–(aq) ⇌ HCO3–(aq) + SO42–(aq)a. H2CO3 and H2SO4b. HCO3– and SO42–c. HCO3– and H2SO4d. Start studying chem 2 test 3 chapter 16. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
[index] [264] [936] [3234] [4074] [4206] [2769] [6769] [4331] [669] [3288]
This acid base equilibrium video tutorial explains how to calculate the pH of a polyprotic acid using ice tables and number lines. It discusses how to calcu... A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion).For the SO4 2- structure use the periodic table to find the total numb... A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion).For the CO3 2- structure use the periodic table to find the total nu... Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com
Copyright © 2024 alltop100casinos.site